Page 28 - วารสารปีที่15ฉบับที่1
P. 28
24
LH-20 ´÷Ë߇ªìπ«—Ø¿“§§ß∑’Ë (stationary phase) Ÿμ√‚§√ß √â“ߢÕß “√π’ȉ¥â®“°°“√ àßμ—«Õ¬à“߇æ◊ËÕ
‚¥¬„™âμ—«∑”≈–≈“¬Õ‘π∑√’¬å∑’Ë¡’¢—È«‡ªìπμ—«™– “√ „π «‘‡§√“–Àå¥â«¬‡∑§π‘§∑“ß ‡ª°‚∑√ ‚§ªï (Spectros-
1
°“√»÷°…“π’È„™â à«πº ¡¢Õß methanol ·≈–πÈ”‡ªìπ copy) ‚¥¬„™â‡§√◊ËÕß H NMR (400 MHz) ·≈–μ√«®
1
«—Ø¿“§‡§≈◊ËÕπ∑’Ë (mobile phase) „π°“√·¬° “√ °—¥ Õ∫πÈ”Àπ—°‚¡‡≈°ÿ≈¥â«¬ GC-MS ¢âÕ¡Ÿ≈ H NMR
acetone æ∫ “√ 5,7,4'-trimethoxyflavone ·≈–πÈ”Àπ—°‚¡‡≈°ÿ≈ Õ¥§≈âÕß°—∫√“¬ß“π«‘®—¬∑’ˉ¥â¡’
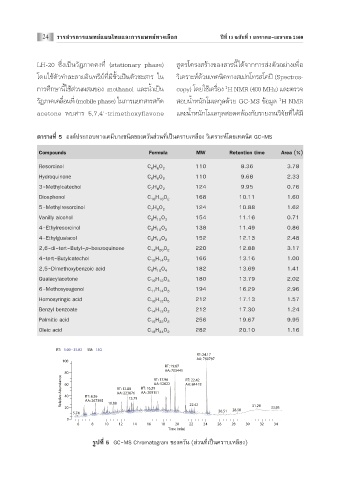
μ“√“ß∑’Ë 5 Õߧåª√–°Õ∫∑“߇§¡’∫“ß™π‘¥¢Õߧ«—π à«π∑’ˇªìπ§√“∫‡À≈◊Õß «‘‡§√“–Àå‚¥¬‡∑§π‘§ GC-MS
Compounds Formula MW Retention time Area (%)
Resorcinol C H O 110 8.36 3.78
6 6 2
Hydroquinone C H O 110 9.68 2.33
6 6 2
3-Methylcatechol C H O 124 9.95 0.76
7 8 2
Diosphenol C H O 168 10.11 1.60
10 16 2
5-Methylresorcinol C H O 124 10.88 1.62
7 8 2
Vanilly alcohol C H O 154 11.16 0.71
8 10 3
4-Ethylresorcinol C H O 138 11.49 0.86
8 10 2
4-Ethylguaiacol C H O 152 12.13 2.48
9 12 2
2,6-di-tert-Butyl-p-benzoquinone C H O 220 12.88 3.17
14 20 2
4-tert-Butylcatechol C H O 166 13.16 1.00
10 14 2
2,5-Dimethoxybenzoic acid C H O 182 13.69 1.41
9 10 4
Guaiacylacetone C H O 180 13.79 2.02
10 12 3
6-Methoxyeugenol C H O 194 16.29 2.96
11 14 3
Homosyringic acid C H O 212 17.13 1.57
10 12 5
Benzyl benzoate C H O 212 17.30 1.24
14 12 2
Palmitic acid C H O 256 19.67 9.95
16 32 2
Oleic acid C H O 282 20.10 1.16
18 34 2
RT: 5.00 - 35.02 SM: 15G
RT: 24.17
AA: 780787
100
RT: 19.67
AA: 703441
80 RT: 17.96 RT: 22.42
Relative Abundance 60 RT: 8.36 RT: 12.88 13.79 RT: 16.29 AA: 52822 AA: 84418
AA: 209151
AA: 223876
40
20 AA: 267395 10.88 22.42 31.28 33.95
26.51 28.50
5.74
0
6 8 10 12 14 16 18 20 22 24 26 28 30 32 34
Time (min)
√Ÿª∑’Ë 5 GC-MS Chromatogram ¢Õߧ«—π ( à«π∑’ˇªìπ§√“∫‡À≈◊Õß)