Page 101 - วารสารกรมการแพทย์แผนไทยฯ ปีที่ 14 ฉบับที่ 3
P. 101
319
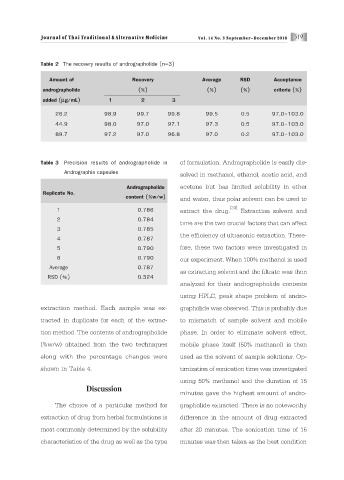
Table 2 The recovery results of andrographolide (n=3)
Amount of Recovery Average RSD Acceptance
andrographolide (%) (%) (%) criteria (%)
added (μg/mL) 1 2 3
26.2 98.9 99.7 99.8 99.5 0.5 97.0-103.0
44.9 98.0 97.0 97.1 97.3 0.5 97.0-103.0
89.7 97.2 97.0 96.8 97.0 0.2 97.0-103.0
Table 3 Precision results of andrographolide in of formulation. Andrographolide is easily dis-
Andrographis capsules
solved in methanol, ethanol, acetic acid, and
Andrographolide acetone but has limited solubility in ether
Replicate No.
content (%w/w) and water, thus polar solvent can be used to
[33]
1 0.786 extract the drug. Extraction solvent and
2 0.784
time are the two crucial factors that can affect
3 0.785
the efficiency of ultrasonic extraction. There-
4 0.787
5 0.790 fore, these two factors were investigated in
6 0.790 our experiment. When 100% methanol is used
Average 0.787
as extracting solvent and the filtrate was then
RSD (%) 0.324
analyzed for their andrographolide contents
using HPLC, peak shape problem of andro-
extraction method. Each sample was ex- grapholide was observed. This is probably due
tracted in duplicate for each of the extrac- to mismatch of sample solvent and mobile
tion method. The contents of andrographolide phase. In order to eliminate solvent effect,
(%w/w) obtained from the two techniques mobile phase itself (50% methanol) is then
along with the percentage changes were used as the solvent of sample solutions. Op-
shown in Table 4. timization of sonication time was investigated
using 50% methanol and the duration of 15
Discussion
minutes gave the highest amount of andro-
The choice of a particular method for grapholide extracted. There is no noteworthy
extraction of drug from herbal formulations is difference in the amount of drug extracted
most commonly determined by the solubility after 20 minutes. The sonication time of 15
characteristics of the drug as well as the type minutes was then taken as the best condition