Page 20 - วารสารกรมการแพทย์แผนไทยฯ ปีที่ 10 ฉบับที่ 2
P. 20
96 «“√ “√°“√·æ∑¬å·ºπ‰∑¬·≈–°“√·æ∑¬å∑“߇≈◊Õ° ªï∑’Ë 10 ©∫—∫∑’Ë 2 情¿“§¡- ‘ßÀ“§¡ 2555
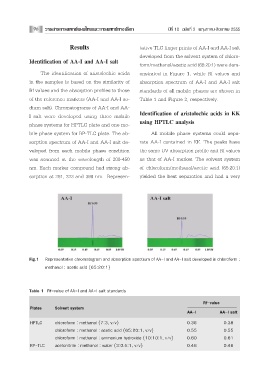
Results tative TLC finger prints of AA-I and AA-I salt
developed from the solvent system of chloro-
Identification of AA-I and AA-I salt
form/methanol/acetic acid (65:20:1) were dem-
The identification of aristolochic acids onstrated in Figure 1, while Rf values and
in the samples is based on the similarity of absorption spectrum of AA-I and AA-I salt
Rf values and the absorption profiles to those standards of all mobile phases are shown in
of the reference markers (AA-I and AA-I so- Table 1 and Figure 2, respectively.
dium salt). Chromatograms of AA-I and AA-
Identification of aristolochic acids in KK
I salt were developed using three mobile
using HPTLC analysis
phase systems for HPTLC plate and one mo-
bile phase system for RP-TLC plate. The ab- All mobile phase systems could sepa-
sorption spectrum of AA-I and AA-I salt de- rate AA-I contained in KK. The peaks have
veloped from each mobile phase condition the same UV absorption profile and Rf values
was scanned at the wavelength of 200-450 as that of AA-I marker. The solvent system
nm. Each marker compound had strong ab- of chloroform/methanol/acetic acid (65:20:1)
sorption at 251, 323 and 398 nm. Represen- yielded the best separation and had a very
Fig.1 Representative chromatogram and absorption spectrum of AA-I and AA-I salt developed in chloroform :
methanol : acetic acid (65:20:1)
Table 1 Rf-value of AA-I and AA-I salt standards
Rf-value
Plates Solvent system
AA-I AA-I salt
HPTLC chloroform : methanol (7:3, v/v) 0.36 0.38
chloroform : methanol : acetic acid (65:20:1, v/v) 0.55 0.55
chloroform : methanol : ammonium hydroxide (10:10:1, v/v) 0.60 0.61
RP-TLC acetonitrile : methanol : water (3:0.5:1, v/v) 0.48 0.46