Page 32 - วารสารการแพทย์แผนไทย ปีที่ 20 ฉบับที่ 2 พฤษภาคม-สิงหาคม 2565
P. 32
230 วารสารการแพทย์แผนไทยและการแพทย์ ทางเลือก ปีที่ 20 ฉบับที่ 2 พฤษภาคม-สิงหาคม 2565
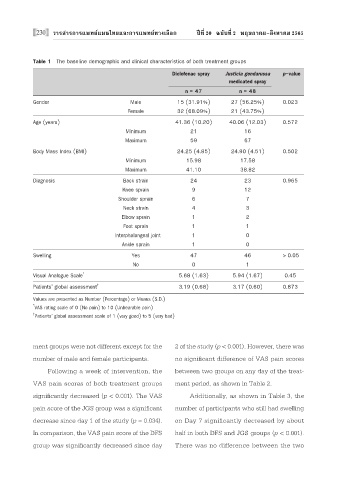
Table 1 The baseline demographic and clinical characteristics of both treatment groups
Diclofenac spray Justicia gendarussa p-value
medicated spray
n = 47 n = 48
Gender Male 15 (31.91%) 27 (56.25%) 0.023
Female 32 (68.09%) 21 (43.75%)
Age (years) 41.36 (10.20) 40.06 (12.03) 0.572
Minimum 21 16
Maximum 59 67
Body Mass Index (BMI) 24.25 (4.85) 24.90 (4.51) 0.502
Minimum 15.98 17.58
Maximum 41.10 38.82
Diagnosis Back strain 24 23 0.965
Knee sprain 9 12
Shoulder sprain 6 7
Neck strain 4 3
Elbow sprain 1 2
Foot sprain 1 1
Interphalangeal joint 1 0
Ankle sprain 1 0
Swelling Yes 47 46 > 0.05
No 0 1
Visual Analogue Scale 5.68 (1.63) 5.94 (1.67) 0.45
*
Patients’ global assessment 3.19 (0.68) 3.17 (0.60) 0.873
†
Values are presented as Number (Percentage) or Means (S.D.)
* VAS rating scale of 0 (No pain) to 10 (Unbearable pain)
† Patients’ global assessment scale of 1 (very good) to 5 (very bad)
ment groups were not different except for the 2 of the study (p < 0.001). However, there was
number of male and female participants. no significant difference of VAS pain scores
Following a week of intervention, the between two groups on any day of the treat-
VAS pain scores of both treatment groups ment period, as shown in Table 2.
significantly decreased (p < 0.001). The VAS Additionally, as shown in Table 3, the
pain score of the JGS group was a significant number of participants who still had swelling
decrease since day 1 of the study (p = 0.034). on Day 7 significantly decreased by about
In comparison, the VAS pain score of the DFS half in both DFS and JGS groups (p < 0.001).
group was significantly decreased since day There was no difference between the two